SeO2 Lewis Structure – How to Draw the Lewis Structure for SeO2
This is Dr. B. Let's do the SeO2 Lewis structure. Se, on the periodic table, 6 valence electrons. Oxygen 6, we've got two of them, for a total
of 18 valence electrons. So we'll put the Se in the center and the
Oxygens on either side. And we'll put two electrons between the atoms
to form the bonds. Around the outside, we have 4, 6, 8, 10, 12,
14, 16, and then two here, 18. We don't have eight valence electrons here,
we don't have an octet for Se. So let's take two electrons from the outside
and move them to the center. We'll just put these two here, right there. That seems to solve the problem.
Now we have 8 valence electrons on the Se,
and the Oxygens, they also have 8. Since we have Se here in this Lewis structure
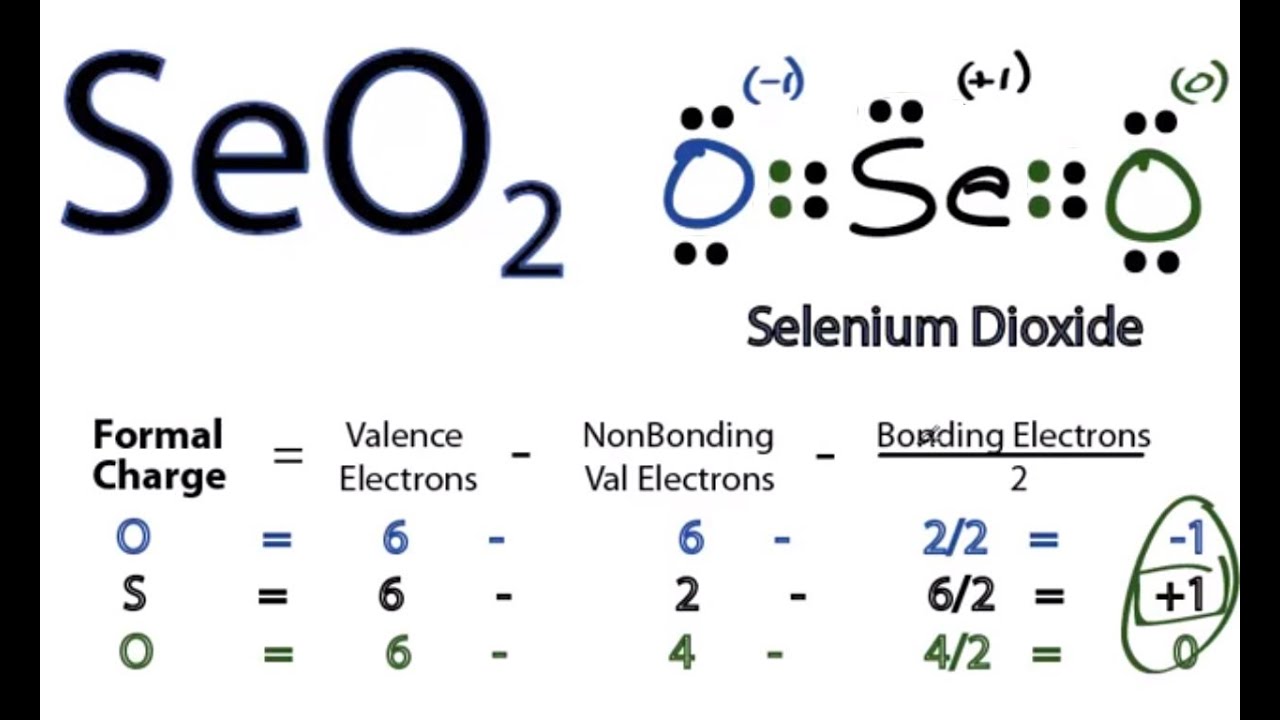
for SeO2, we'll probably want to check the formal charges to make sure that this is the
best structure for SeO2. So let's do that. So this Oxygen right here, 6 valence electrons
on the periodic table. Six that are nonbonding, minus two that are
bonding. So 6 – 6 – 1 is -1. For the Se, we have 6 valence electrons. We have 2 that are nonbonding, and then we
have 2 plus 4 that are bonding; six. So 6 – 2 – 3 is a positive 1. And finally, for this Oxygen over here, we
have 6 valence electrons, minus the 4 nonbonding, minus 4 bonding divided by 2. Six minus 4 minus 2 is zero. Because I see these positive and negative
charges here, I think this might not be the best structure. When I see a +1 here, that makes me think
I'm going to need to add another double bond. So what I'll do is, I'll take these two here
and share them right here and recalculate my formal charges.

So by adding this double bond here, I now
see that my formal charges are all zero. I've recalculated all these numbers. Because of that, that tells me that this is
the best Lewis structure for SeO2. This is Dr. B., and thanks for watching..